The Expanding Analog Ban: 2025’s Defining Regulatory Trend
Throughout 2025, a dramatic shift is unfolding across the United States: a rapidly growing number of states are banning or severely restricting hemp-derived cannabinoids like HHC (Hexahydrocannabinol), THCP (Tetrahydrocannabiphorol), THC‑O (THC-O Acetate), and other semi- or fully-synthetic analogs. As states race ahead of federal action, these new laws threaten to upend national product lines and R&D strategies. This post delivers a practical, state-level tracker and compliance playbook for commercial formulators.
Why the Crackdown Now?
Driven by surges in intoxicating, hemp-derived edibles and vaping products, state legislatures are rewriting their definitions of banned substances. Most are no longer content with targeting just Delta-8 THC; newly adopted terms such as “analog,” “isomer,” and “chemically modified” now sweep in entire categories of cannabinoids, including those with zero naturally occurring presence in cannabis plants. Others, like Utah and New Mexico, focus on “naturally derived, non-intoxicating” standards. Still more use catch-all language legally tethered to any substance with similar psychoactive effect or structure as Delta-9 THC.
States are justifying these steps by citing consumer safety, the difficulty of distinguishing synthetic and semi-synthetic products, and the patchwork created by the 2018 Farm Bill. The implication for manufacturers and researchers is clear: proactive compliance is now business-critical.
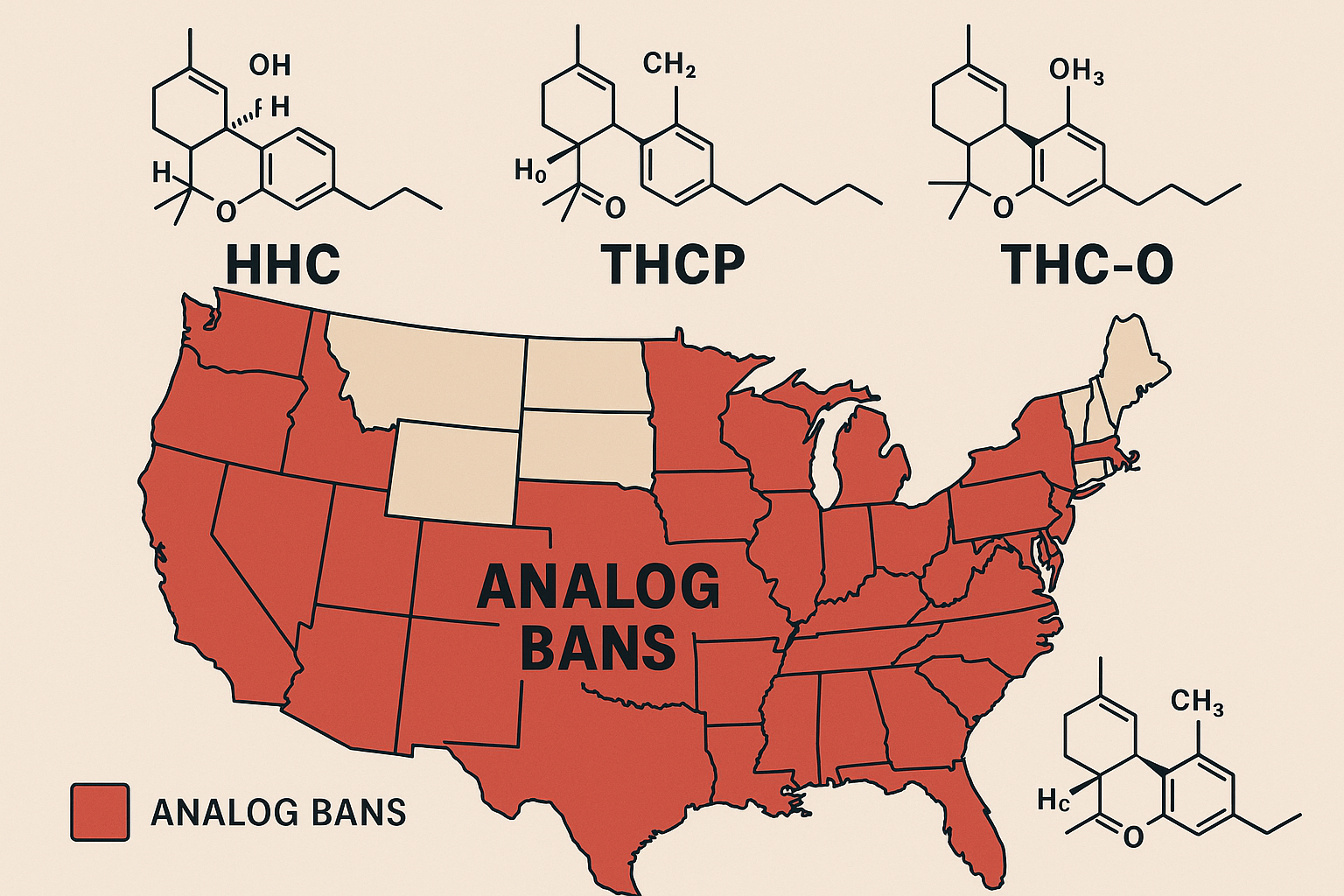
2025: A 50-State Analog Ban & Restriction Tracker
Note: Laws evolve rapidly. Always verify with the relevant state agency or contact CannabisRegulations.ai for real-time updates.
States with Express Bans or Severe Restrictions (as of September 2025)
- Utah: Bans all cannabinoids “chemically converted or synthesized.” Further restricts sale to non-intoxicating, naturally derived compounds only. Utah 2025 hemp amendments.
- New Mexico: Emergency regulations (Aug 2025) ban manufacture and sale of all synthetic cannabinoids, regardless of origin.[PDF]
- Texas: SB 5 prohibits any hemp product with a “detectable amount of any cannabinoid” not naturally occurring in intact hemp. No longer relies only on delta-9 THC threshold. Synthetic cannabinoid update.
- California: Ban on all intoxicating hemp-derived THC analogs—adults-only dispensaries only, with expanded enforcement and seizure protocols. California 2025 update
- Maryland: Effective July 1, 2025, all THC products must meet enhanced safety, labeling, and provenance standards—ban on synthetic analogs.
- Oregon, Colorado, Nevada: All have codified bans or strict scheduling of THC analogs and isomers.
- Alaska, Delaware, Hawaii, Idaho, Mississippi, Montana, North Dakota, New York, Rhode Island, Vermont, Washington, West Virginia: All with enacted bans or analog scheduling covering most synthetic/semi-synthetic cannabinoids, including HHC, THCP, THC-O.
States with Partial Bans, Thresholds, or Catch-All Provisions
- Iowa, Kansas, Louisiana, Michigan, Minnesota, New Hampshire, Virginia: Restrict cannabinoids by total THC threshold per serving or package, with catch-all rules that can sweep in analogs and isomers.
- Kentucky: HHC currently legal (as of September 2025), but pending legislation could add it and other intoxicating cannabinoids to state ban lists. HHC State Map
States with No Explicit Analog Bans (but regulatory change is likely)
- Alabama, Florida, Maryland, Wyoming: Have imposed youth access bans; no broad analog ban yet, but regulatory proposals are under review.
- Federal Outlook: Proposed federal legislation would potentially ban most cannabinoids "manufactured outside of the cannabis plant" (i.e., any non-naturally occurring analog), affecting nearly all market SKUs. Federal hemp bill insights
Key Compliance Triggers: Labeling, Ingredient Provenance, Enforcement
Labeling & Threshold Triggers
- “THC” or “Total THC” Calculations: Most states require listing all major and minor THC isomers; some now include analogs or derivatives, not just delta-9.
- Intoxication-Based Restrictions: Some states require special labeling (or outright bans) for anything deemed “intoxicating”—not just via chemical structure, but also psychoactive effect or consumer reports.
- CBD:THC Ratio and Non-Intoxicating Claims: Emerging rules in several states tie legality to a CBD:THC ratio floor, or require testing to prove that a SKU is non-intoxicating.
- Provenance Documentation: States may now require certified documentation of ingredient origin, reaction pathway (extraction/conversion steps), and attestation of "naturally derived" status.
Penalties and Enforcement
Penalties for violations range from product seizure and administrative fines to license suspension/revocation, felony charges for knowing manufacture or sale of a banned compound, and even civil liability for consumer harm.
- Texas: Summary product seizure, fines, mandatory recall, and criminal charges under state controlled substance laws.
- California: Enforcement includes coordinated inspections, seizure/destruction, and fines that may exceed $10,000 per SKU per violation.
- Utah & New Mexico: Administrative fines and license revocation; repeat offenses may lead to business closure.
Navigating the analog ban landscape requires rigorous product development processes and rapid market segmentation. Here’s a field-tested compliance checklist:
1. Ingredient Provenance & Reaction Pathway Records
- Obtain certificates of analysis and provenance from upstream suppliers. For all cannabinoids, retain detailed documentation (COA, batch records, conversion steps).
- Clearly disclose in technical documents whether each ingredient is plant-extracted, naturally derived, chemically converted (from CBD or other cannabinoids), or purely synthetic.
2. Intoxication and Psychoactivity Screening
- Implement testing to measure not just delta-9-THC, but all psychoactive analogs, with a focus on demonstrating true “non-intoxicating” profiles where possible.
- Establish and record CBD:THC ratios in end products; maintain supporting evidence in case of audit.
3. SKU Risk Segmentation by State Tier
- Tier 1 (High Risk): States with express analog/synthetic bans—remove affected SKUs or reformulate to comply (e.g., remove HHC, THCP, THC-O).
- Tier 2 (Medium/Transitional Risk): States with catch-all language or threshold-based triggers—consider lowering total THC, ensuring non-intoxicating profiles, or labeling with prominent warnings.
- Tier 3 (Currently Permissive): States with no broad ban—reassess monthly for legislative updates; prepare contingency reformulations and alternate packaging for rapid compliance.
4. Labeling and Batch Documentation
- Update all packaging to comply with each state’s definition of “THC,” isomer, and analog disclosures.
- Add QR codes or online documentation for public/agency viewing of COAs, ingredient origin, and compliance attestations.
- Develop region-specific SKUs with alternate cannabinoid profiles (e.g., CBD-only, CBG-enriched, or THC-compliant ratios).
- For HHC, THCP, THC-O, and similar analogs—phase out or substitute with compliant non-intoxicating cannabinoids where possible.
6. Proactive Agency Notices & Legal Monitoring
- Routinely submit new product documentation to relevant state agencies before launch—seek agency review if thresholds or analog status is not clear.
- Subscribe to regulatory update services; assign compliance staff to monitor bills and emergency rules in every active market.
Takeaways for Stakeholders
- Analog bans in 2025 will force profound supply chain and R&D adaptations. A conservative approach based on ingredient traceability and state-by-state segmentation is now essential for all hemp/cannabis brands.
- Non-compliance is no longer a civil risk alone: expect coordinated enforcement, license actions, and potential criminal prosecution in key states.
- The days of "one formulation for all US markets" are rapidly ending—building a compliance-first product roadmap and labeling strategy is the new industry standard.
For real-time state bans, threshold triggers, and operational guidance, stay ahead with CannabisRegulations.ai—the leading resource for cannabinoid compliance, licensing, and R&D support in the evolving regulatory landscape.